MOLECULAR UNDERPINNINGS OF GENERAL ANESTHESIA
Modulation of voltage gated sodium channels is thought to play a major role in the pharmacologically induced state of
general anesthesia; indeed, several members of this class of channels show a significant response to general anesthetics.
However, the detailed mechanism of inhibition or potentiation of these channels is completely unknown. Recently, the
structures of several bacterial orthologs became available offering the opportunity to shed some light on this issue.
In collaboration with the groups of Manuel Covarrubias from Jefferson University (electrophysiology) and Roderic
Eckenhoff from University of Pennsylvania (anesthesiology), we have identified binding sites and access pathways for
the volatile general anesthetics isoflurane and sevoflurane for one of these bacterial voltage gated sodium channels,
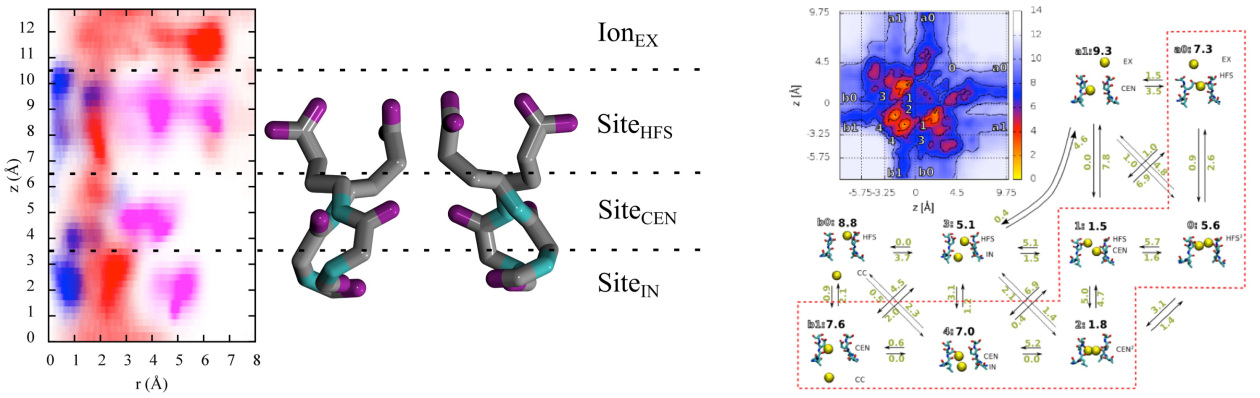
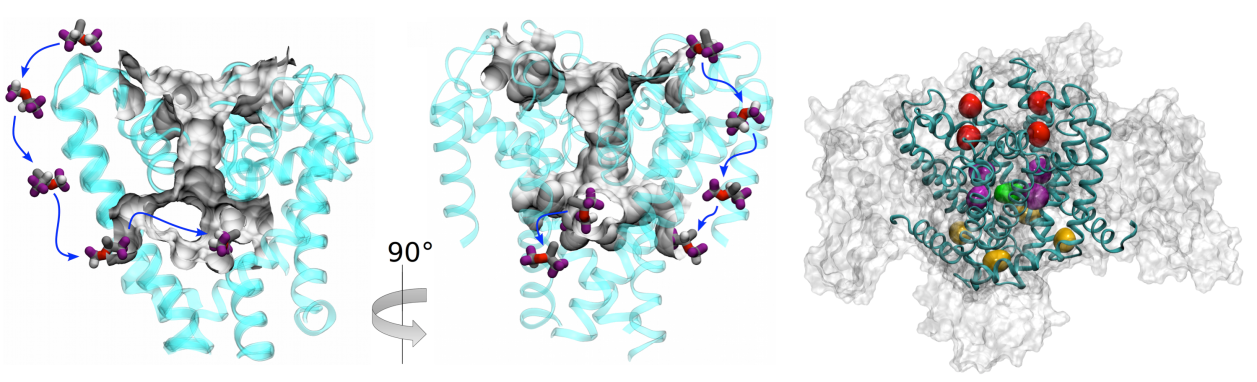
NaChBac. Isoflurane and sevoflurane binds the channel at physiologically relevant concentrations in three distinct
locations. One site is in the pore of the channel, suggesting that general anesthetics may hinder permeation of sodium
ions, while the other two are distant from the conduction pathway and affect conduction via allosteric modulation.
We are currently exploring the effect of drug binding on the stability of the non-conductive (inactivated) structural
states of the channel to obtain a quantitative thermodynamic model of ion current modulation by general anesthetics.
The exciting perspective is to translate this information to the entire class of pharmacologically relevant mammalian
channels present in the axons of neurons and generate a physically based mesoscopic model to study the action of
general anesthetics in relevant neural networks.
M. Covarrubias, A. F Barber, V. Carnevale, W. Treptow, and R. G Eckenhoff. Mechanistic insights into the modulation of
voltage-gated ion channels by inhaled anesthetics. BIOPHYSICAL JOURNAL, 109:10, 2003-2011, 2015.
A. Barber, V. Carnevale, M. L. Klein, R. G. Eckenhoff, M. Covarrubias Modulation of a voltage-gated Na+channel by
sevoflurane involves multiple sites and distinct mechanisms.
PROC. NATL. ACAD. SCI. USA, doi: 10.1073/pnas.1405768111.
S. G. Raju, A. B. Barber, D. LeBard, M. L. Klein, V. Carnevale Exploring Volatile General Anesthetic Binding to a
Closed Membrane-Bound Bacterial Voltage-Gated Sodium Channel via Computation PLOS COMPUTATIONAL
BIOLOGY doi:10.1371/journal.pcbi.1003090 (2013).